💉💉💉 A 3rd COVID-19 jab...
- Alireza FakhriRavari, PharmD, BCPS, BCIDP, AAHIVP

- Aug 13, 2021
- 1 min read

Last night (8/12/21), the FDA extended the emergency use authorizations (EUA) for Pfizer-BioNTech's BNT162b2 (currently for age 12 years and older) and Moderna's mRNA-1273 (currently for age 18 years and older) to allow for the use of an additional dose in certain immunocompromised individuals, such as solid organ transplant recipients or those who are diagnosed with conditions that are considered to have an equivalent level of immunocompromise (https://bit.ly/37DWxk3).
Today (8/13/21), the CDC's ACIP voted 11-0 to approve a 3rd dose following a primary 2-dose series in immunocompromised people. Of note, whenever possible, vaccination should be given at least 2 weeks prior to initiation of immunosuppressive therapies. Who's considered immunocompromised? Here's a slide from ACIP meeting:
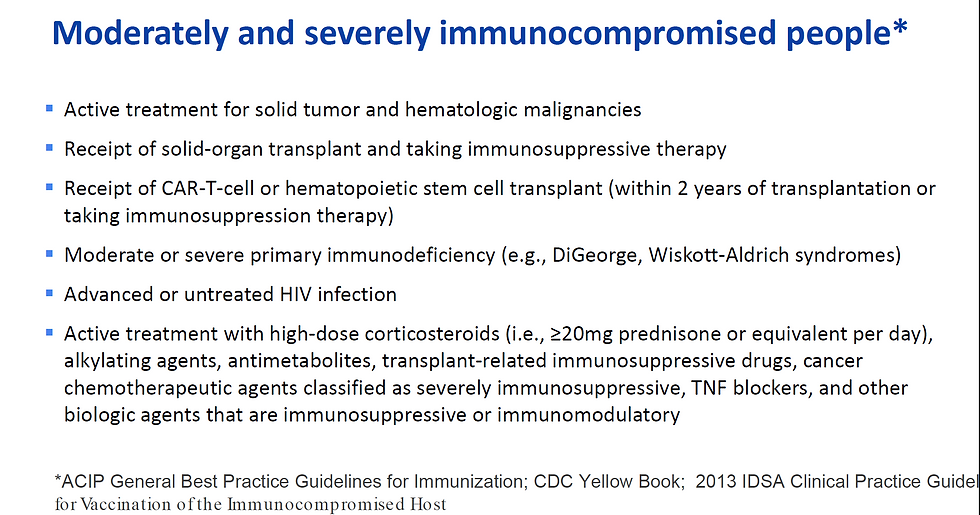
What about individuals who received a single dose of Johnson & Johnson's Ad26.COV2.S vaccine? No recommendations yet due to lack of data, but probably best to get at least one mRNA vaccine booster.







Commentaires